
Mission
BioSentinel develops innovative tools and reagents for the study of botulinum neurotoxin (BoNT). Our tests and assays set new standards for speed, sensitivity, and accuracy while at the same time lowering testing costs and removing animal usage.
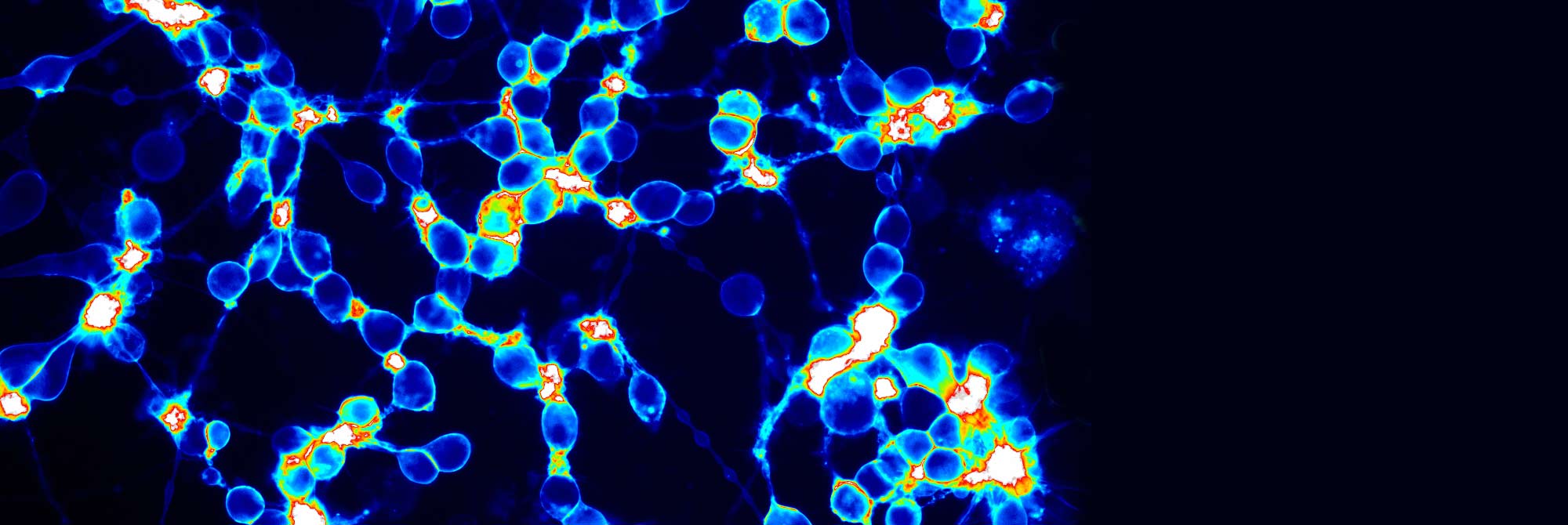
Our assays detect BoNT in hours with sensitivities that rival that of the mouse bioassay, the “gold standard” for BoNT detection. The assays are easy to use, readily adaptable to various applications, and in the case of the cell-based assay, can directly replace the laborious mouse bioassay.
Our laboratory has been creating technology advancements for improved BoNT research and detection since 2007. We offer a suite of assays for detection and quantification of BoNT in diverse samples such as food, serum, purified toxin, and drug product samples, and the expertise to match you with the right product to further your research.
The Company
BioSentinel, Inc. (BioSentinel) designs rapid, sensitive tests for the detection of botulinum neurotoxins (BoNTs) as required by the US Department of Defense and the Department of Homeland Security to protect the nation from intentional misuse of these Class A bioterrorism agents. BioSentinel's proprietary rapid detection technology also addresses the needs of pharmaceutical companies who sell botulinum neurotoxin for FDA-approved cosmetic and therapeutic applications.
BioSentinel has a vigorous research and development program that includes a state-of-the-art laboratory in Madison, WI, and partners throughout the US and worldwide. BioSentinel maintains active collaborations with the National Wildlife Health Center, the US Army Medical Research Institute of Chemical Defense (Aberdeen Proving Grounds, MD), and the University of Wisconsin-Madison. BioSentinel is also partnered with private companies who share BioSentinel's goals of developing innovative technologies/platforms for the detection, characterization, and quantification of BoNTs.
The BioSentinel team is uniquely qualified to design BoNT detection technologies for both biodefense programs and pharmaceutical uses and applications. Read more about Our Team.
